Your doctor will decide whether TAZVERIK® may be a treatment option for you. Always follow your doctor's instructions about how to take TAZVERIK.
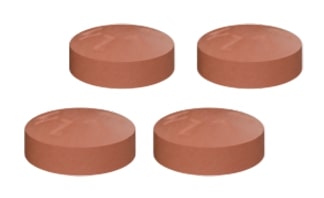
The recommended dosing is 4 tablets (200 mg each), twice daily.
Your doctor will confirm what your dose should be, as some patients may need a different dose.
1st dose

2nd dose

The recommended dosing is 4 tablets (200 mg each), twice daily.
Your doctor will confirm what your dose should be, as some patients may need a different dose.

1st dose

2nd dose

Before taking TAZVERIK tell your healthcare provider about all of your medical conditions, including if you:
- Are pregnant or plan to become pregnant. TAZVERIK can harm your unborn baby. Your healthcare provider will give you a pregnancy test before you start treatment with TAZVERIK. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- Females who are able to become pregnant should use effective non-hormonal birth control (such as condoms) during treatment and for 6 months after the final dose of TAZVERIK. Birth control pills (oral contraceptives) and other hormonal forms of birth control may not be effective if used during treatment with TAZVERIK. Talk to your healthcare provider about birth control options that are right for you.
- Males with female partners who are able to become pregnant should use effective birth control during treatment and for 3 months after the final dose of TAZVERIK.
- Are breastfeeding or plan to breastfeed. It is not known if TAZVERIK passes into your breast milk. Do not breastfeed during treatment and for 1 week after the final dose of TAZVERIK.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TAZVERIK may affect the way other medicines work and other medicines may affect how TAZVERIK works.
What should I avoid while taking TAZVERIK?
- Avoid eating grapefruit or drinking grapefruit juice during treatment with TAZVERIK.
- Avoid taking St. John’s wort during treatment with TAZVERIK.
Talk to your healthcare provider before starting any new medications, vitamins, or herbal supplements.