

Recommended dose of 800 mg (4 x 200 mg tablets) taken orally, twice daily until disease progression or unacceptable toxicity1
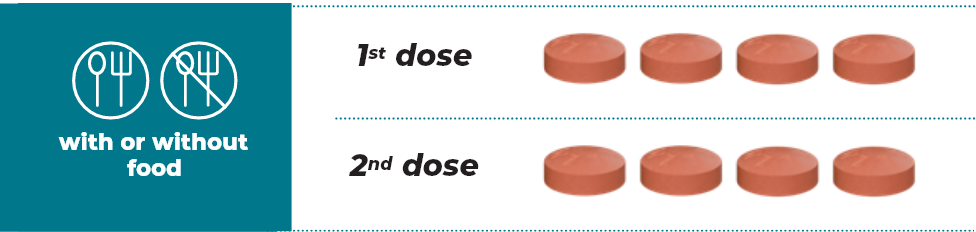

*Tablets not actual size


Swallow tablets whole. Do not cut, crush, or chew tablets. Do not take an additional dose if a dose is missed or vomiting occurs after taking TAZVERIK®, but continue with the next scheduled dose.1
NDC number (10 digit): 72607-100-00 NDC number (11 digit): 72607-0100-00 How supplied: 240-count bottle NDC= National Drug Code
NDC number (10 digit): 72607-100-00 NDC number (11 digit): 72607-0100-00 How supplied: 240-count bottle NDC= National Drug Code
Recommended dose reductions of TAZVERIK for adverse reactions1
| DOSE | DOSAGE |
|---|---|
| First | 600 mg twice daily |
| Second | 400 mg twice daily* |
*Permanently discontinue TAZVERIK in patients who are unable to tolerate 400 mg orally twice daily.1
Recommended dosage modifications of TAZVERIK for adverse reactions1
| ADVERSE REACTION & SEVERITY | DOSAGE MODIFICATION |
|---|---|
| Neutropenia Neutrophil count less than 1 x 109/L |
|
| Thrombocytopenia Platelet count less than 50 x 109/L |
|
| Anemia Hemoglobin less than 8 g/dL |
|
| Other adverse reactions Grade 3 |
|
| Other adverse reactions Grade 4 |
|
Recommended dose reductions of TAZVERIK for moderate CYP3A inhibitors where coadministration cannot be avoided1
| CURRENT DOSAGE | ADJUSTED DOSAGE |
|---|---|
| 800 mg orally twice daily | 400 mg orally twice daily |
| 600 mg orally twice daily | 400 mg for first dose and 200 mg for second dose |
| 400 mg orally twice daily | 200 mg orally twice daily |
| NO DOSE ADJUSTMENTS ARE RECOMMENDED FOR PATIENTS WITH: |
|---|
|
•
Mild to severe renal impairment, including end-stage renal disease1 |
|
•
Mild hepatic impairment. TAZVERIK has not been studied in patients with moderate or severe hepatic impairment1† |
†Mild=total bilirubin > 1 to 1.5 times ULN or AST > ULN; moderate=total bilirubin > 1.5 to 3 times ULN; severe=total bilirubin > 3 times ULN.1
CYP3A=Cytochrome P450 (CYP)3A; AST=aspartate aminotransferase; ULN=upper limit of normal.
Reference: 1. TAZVERIK (tazemetostat) Prescribing Information. Cambridge, MA: Epizyme, Inc., December 2023.
