CLINICAL TRIAL OVERVIEW
TAZVERIK® efficacy and safety were evaluated in an open-label, single-arm cohort (Cohort 5) of a multicenter study in patients with histologically confirmed, metastatic or locally advanced epithelioid sarcoma (n=62). The primary efficacy endpoint was the overall response rate (ORR) and a secondary efficacy endpoint was duration of response (DOR).1,2
TAZVERIK DEMONSTRATED EFFICACY IN EPITHELIOID SARCOMA TRIAL

15% of all patients studied
achieved an ORR
(95% CI: 7-26%; n=9/62)1,2,*

67% of all responders (complete or partial)
achieved a DOR ≥ 6 months
(range=3.7-24.5+ months)1
Time to response ranged from 1.4 to 18.4 months.1
*The ORR included 1.6% (n=1/62) of patients with a complete response and 13% (n=8/62) with a partial response.1,2
Exploratory Subgroup Analysis Based on Investigator Assessment†

TAZVERIK as first-line systemic therapy2
- ORR was 25% (95% CI: 10-47; n=6/24)
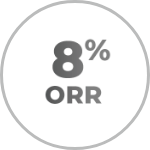
TAZVERIK in later lines2
- ORR was 8% (95% CI: 2-21; n=3/38)

TAZVERIK as first-line systemic therapy2
- Median DOR was 9.5 months (95% CI: 7.9 months-NE)
TAZVERIK in later lines2
- Median DOR was not yet reached at follow-up of 2 years (95% CI: 9.2 months-NE)
LIMITATIONS: These additional data from the phase 2, single-arm study represent subgroup analyses of overall response rate and duration of response. No conclusions of statistical significance can be drawn from these subgroup analyses.
Tazemetostat (TAZVERIK®) is included in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcomas with an NCCN category 2A recommendation as the only preferred regimen option for appropriate patients with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection*
aStudy design: TAZVERIK was studied in the largest prospective, open-label, single-arm, multicenter phase 2 study in patients with histologically confirmed, metastatic or locally advanced epithelioid sarcoma. Patients received 800 mg of TAZVERIK orally twice daily until confirmed disease progression or unacceptable toxicity. The major efficacy endpoints included confirmed overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 as assessed by blinded independent central review (BICR) and duration of response. The median duration of follow-up at the time of the efficacy analysis was 14 months (range 0.4 to 31). Among the 62 patients who received TAZVERIK, all had INI1 loss; median age was 34 years (range 16 to 79); 63% were male, 76% were White, 11% were Asian, 44% had proximal disease, 92% had an ECOG PS of 0 or 1, and 8% had an ECOG PS of 2. Prior surgery occurred in 77% of patients; 61% received prior systemic chemotherapy.1,3
*Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma V.4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 03, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CI=confidence interval; DOR=Duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; NE=not estimable; ORR=overall response rate.
References: 1. TAZVERIK (tazemetostat) Prescribing Information. Cambridge, MA: Epizyme, Inc., August 2024. 2. Gounder M, Schöffski P, Jones RL, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Onc. 2020;21(11):1423-1432. 3. Data on file.

