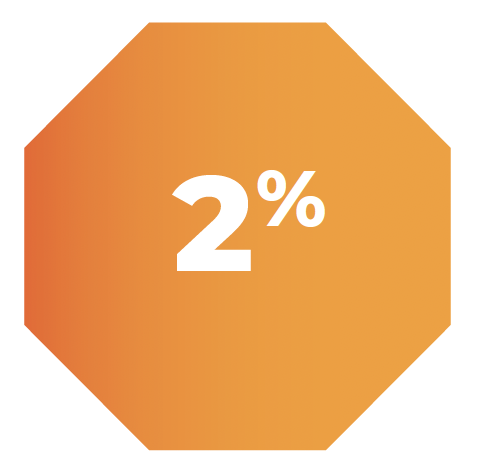
2% of patients, 1/62, required permanent discontinuation of treatment due to an adverse reaction of altered mood.1

2% of patients, 1/62, required a dose reduction due to decreased appetite.1
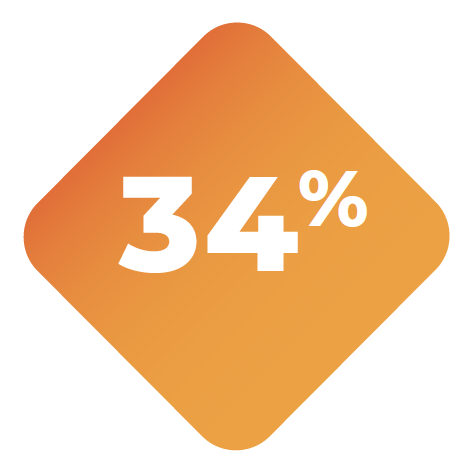
34% of patients required dose interruptions due to adverse reactions.
The most frequent adverse reactions requiring dose interruptions in ≥3% of patients were hemorrhage, increased ALT, and increased AST.1
Serious adverse reactions occurred in 37% of patients who received TAZVERIK®. Serious adverse reactions in ≥3% of patients who received TAZVERIK were hemorrhage, pleural effusion, skin infection, dyspnea, pain, and respiratory distress.1
The most common adverse reactions (≥20%) were pain (52%), fatigue (47%), nausea (36%), decreased appetite (26%), vomiting (24%), and constipation (21%).1
ALT=alanine aminotransferase; AST=aspartate aminotransferase.
Adverse Reactions (≥10%) in Patients with Epithelioid Sarcoma Treated with TAZVERIK (N=62)1
| ADVERSE REACTIONS | ALL GRADES (%) | GRADE 3 OR 4 (%) |
|---|---|---|
| General | ||
| Paina | 52 | 7 |
| Fatigueb | 47 | 1.6 |
| Gastrointestinal | ||
| Nausea | 36 | 0 |
| Vomiting | 24 | 0 |
| Constipation | 21 | 0 |
| Diarrhea | 16 | 0 |
| Abdominal painc | 13 | 1.6 |
| Metabolism and nutrition | ||
| Decreased appetite | 26 | 4.8 |
| Respiratory, thoracic and mediastinal | ||
| Cough | 18 | 0 |
| Dyspnead | 16 | 4.8 |
| Vascular | ||
| Hemorrhagee | 18 | 4.8 |
| Nervous system | ||
| Headache | 18 | 0 |
| Investigations | ||
| Weight decreased | 16 | 7 |
- Includes tumor pain, pain in extremity, non-cardiac chest pain, flank pain, back pain, arthralgia, bone pain, cancer pain, musculoskeletal pain, myalgia, neck pain1
- Includes fatigue and asthenia1
- Includes abdominal pain, gastrointestinal pain, abdominal pain lower1
- Includes dyspnea and dyspnea exertional1
- Includes wound hemorrhage, rectal hemorrhage, pulmonary hemorrhage, hemorrhage intracranial, cerebral hemorrhage, hemoptysis1
Select Laboratory Abnormalities (≥10%) Worsening From Baseline in Patients With Epithelioid Sarcoma Treated With TAZVERIK1
| LABORATORY ABNORMALITY | TAZVERIK* | ||
|---|---|---|---|
| ALL GRADES (%) | GRADE 3 OR 4 (%) | ||
| Hematology | |||
| Decreased hemoglobin | 49 | 15 | |
| Decreased lymphocytes | 36 | 13 | |
| Decreased white blood cell count | 19 | 0 | |
| Chemistry | |||
| Increased triglycerides | 36 | 3.3 | |
| Increased glucose | 33 | 1.6 | |
| Decreased sodium | 30 | 1.7 | |
| Decreased phosphate | 28 | 1.7 | |
| Decreased albumin | 23 | 0 | |
| Increased alkaline phosphatase | 23 | 1.7 | |
| Decreased potassium | 20 | 1.7 | |
| Increased aspartate aminotransferase | 18 | 3.5 | |
| Decreased calcium | 16 | 0 | |
| Decreased glucose | 16 | 0 | |
| Increased partial thromboplastin time | 15 | 5 | |
| Increased alanine aminotransferase | 14 | 3.4 | |
| Increased creatinine | 12 | 0 | |
| Increased potassium | 12 | 0 | |
*The denominator used to calculate the rate varied from 39 to 61 based on the number of patients with a baseline value and at least one post-treatment value.
TAZVERIK does not require special monitoring for laboratory abnormalities.
Reference: 1. TAZVERIK (tazemetostat) Prescribing Information. Cambridge, MA: Epizyme, Inc., August 2024.
