Recommended dose of 800 mg (4 x 200 mg tablets) taken orally, twice
daily, until disease progression or unacceptable toxicity.1

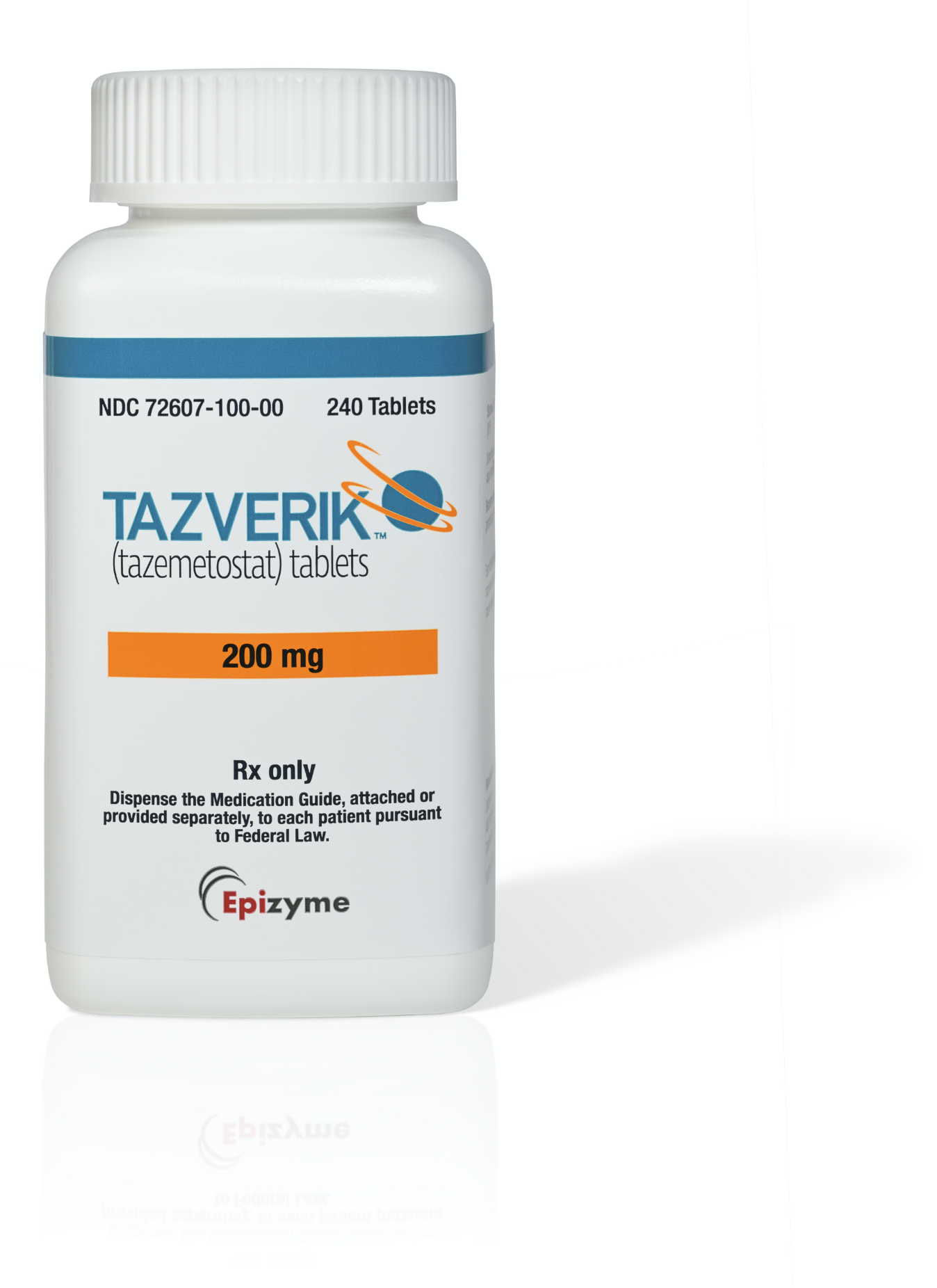
NDC number (10 digit):
72607-100-00
NDC number (11 digit):
72607-0100-00
How supplied:
240-count bottle
NDC=National Drug Code


1st dose

Tablets are not
actual size.
2nd dose

Swallow tablets whole. Do not cut, crush, or chew tablets.
Do not take an additional dose if a dose is missed or vomiting occurs after taking TAZVERIK, but continue with the next scheduled dose.1
TAZVERIK works through an epigenetic mechanism. It may take time for your patients to respond to treatment1
- For patients who experienced an overall response in the clinical trial, the median time to response was:
3.9 months (range: 1.6 to 16.3) for patients with EZH2 WT.1
and
3.7 months (range: 1.6 to 10.9) for patients with EZH2 MT.1
Recommended dose of 800 mg (4 x 200 mg tablets) taken orally, twice daily, until disease progression or unacceptable toxicity.1

1st dose

Tablets are not actual size.
2nd dose

Swallow tablets whole. Do not cut, crush, or chew tablets.
Do not take an additional dose if a dose is missed or vomiting occurs after taking TAZVERIK, but continue with the next scheduled dose.1
NDC number (10 digit): 72607-100-00
NDC number (11 digit): 72607-0100-00
How supplied: 240-count bottle
NDC=National Drug Code
TAZVERIK works through an epigenetic mechanism. It may take time for your patients to respond to treatment1
For patients who experienced an overall response in the clinical trial, the median time to response was:
3.9 months (range: 1.6 to 16.3) for patients with EZH2 WT.1
and
3.7 months (range: 1.6 to 10.9) for patients with EZH2 MT.1
EZH2=enhancer of zeste homolog 2; WT=wild type; MT=mutant type.
Reference: 1. TAZVERIK (tazemetostat) Prescribing Information. Cambridge, MA: Epizyme, Inc., August 2024.
