A closer look at how TAZVERIK works
A closer look at how TAZVERIK works
A closer look at how TAZVERIK works
Watch the unique mechanism of action to see how TAZVERIK can help reduce aberrant B-cell proliferation, associated with the development of FL.1,2
This information is derived from animal studies and does not demonstrate clinical efficacy or safety.1,3
EZH2 plays a critical role in normal B-cell development1,3-5
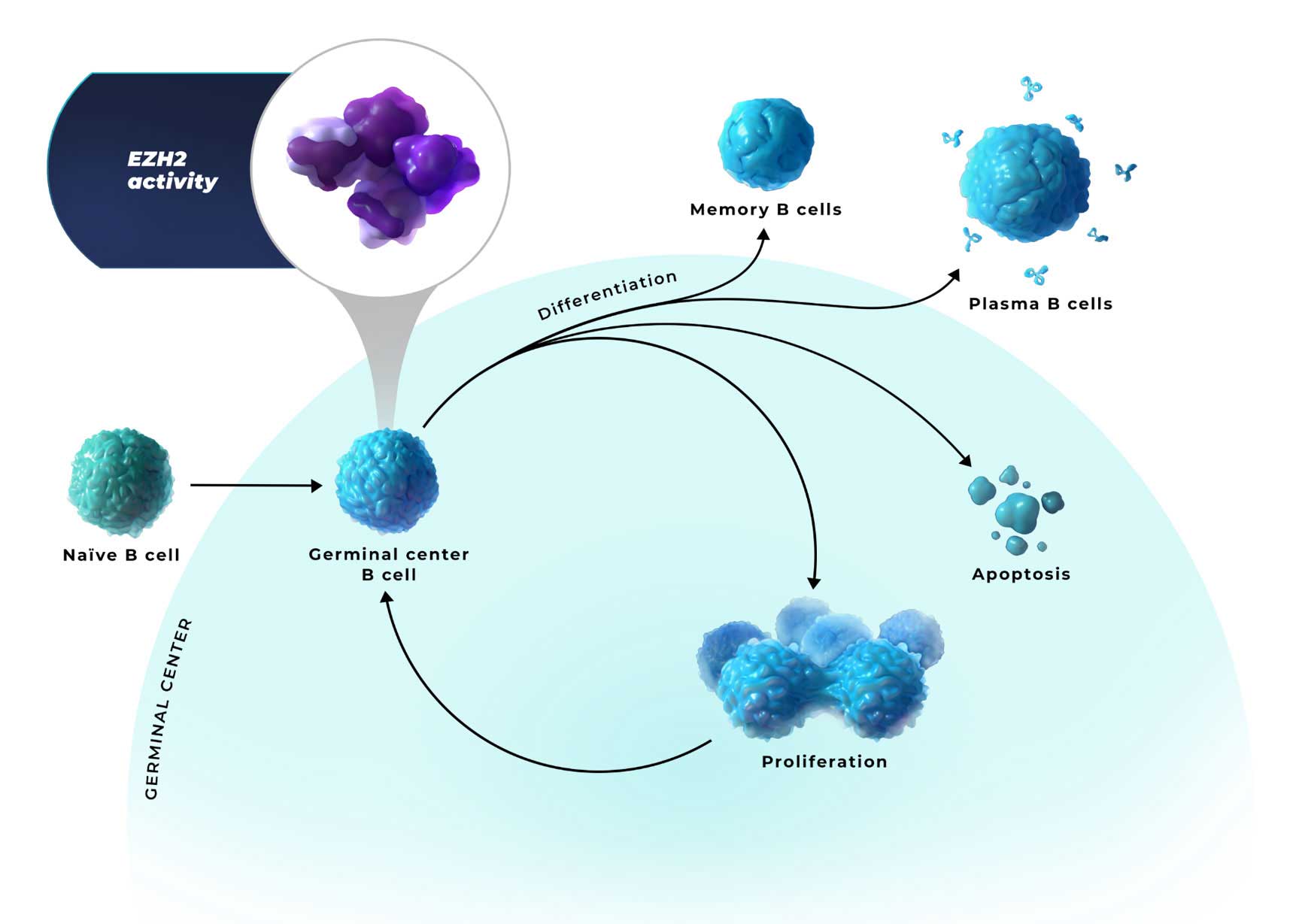
EZH2 is an epigenetic regulator of
B-cell identity in the germinal center1,3
- EZH2 activity represses the expression of genes involved in differentiation, negative cell cycle regulation, and apoptosis. This allows B cells to proliferate and survive1,4,6
- In healthy cells, EZH2 activity is subsequently downregulated to allow for B-cell differentiation and apoptosis6,7
EZH2=enhancer of zeste homolog 2.
EZH2 plays a critical role in follicular lymphoma1,3-5
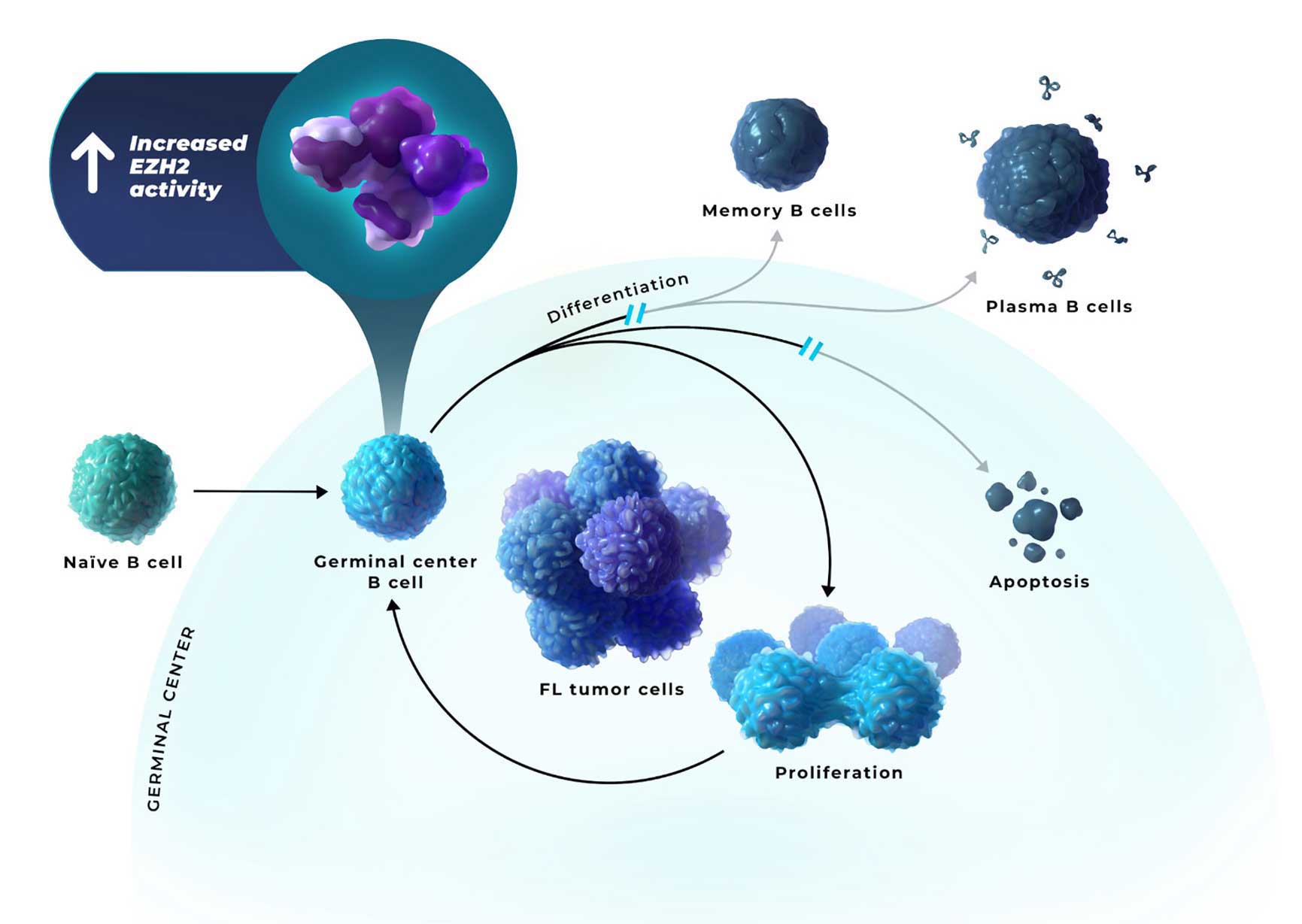
B cells locked in a proliferative germinal center state can lead to the accumulation of malignant B cells and the development of FL8
- FL is caused by heterogenous combinations of oncogenic hits, leading to high EZH2 activity, which can enable persistent epigenetic silencing of the genes involved in differentiation, negative cell cycle regulation, and apoptosis1,4-6
EZH2=enhancer of zeste homolog 2.
TAZVERIK can help reduce aberrant B-cell proliferation associated with the development of FL1-5,8-10
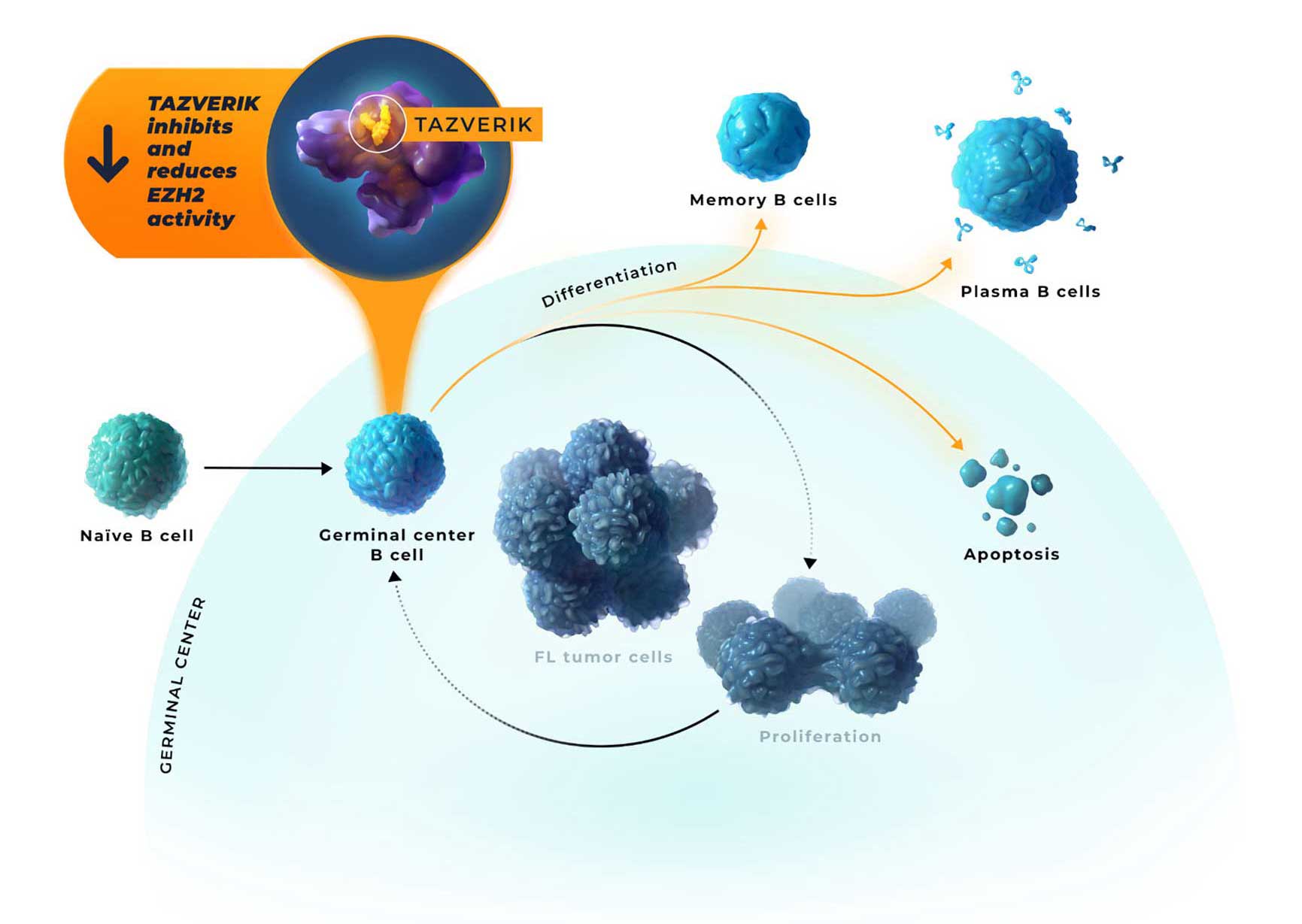
- Inhibition of EZH2 activity by TAZVERIK may enable epigenetic expression of genes that allow for germinal center exit1,2,11
- Regardless of oncogenic mutation, follicular lymphoma tumors have a critical dependence on EZH2 for growth and survival2,12
This information is derived from animal studies and does not demonstrate clinical efficacy or safety.1,3
EZH2=enhancer of zeste homolog 2.
References: 1. Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23(5):677-692. doi:10.1016/j.ccr.2013.04.011 2. TAZVERIK (tazemetostat) Prescribing Information. Cambridge, MA: Epizyme, Inc., August 2024. 3. Velichutina I, Shaknovich R, Geng H, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116(24):5247-5255. doi:10.1182/blood-2010-04-280149 4. Huet S, Sujobert P, Salles G. From genetics to the clinic: a translational perspective on follicular lymphoma. Nat Rev Cancer. 2018;18(4):224-239. doi:10.1038/nrc.2017.127 5. Lackraj T, Goswami R, Kridel R. Pathogenesis of follicular lymphoma. Best Pract Res Clin Haematol. 2018;31(1):2-14. doi:10.1016/j.beha.2017.10.006 6. Lue JK, Amengual JE. Emerging EZH2 inhibitors and their application in lymphoma. Curr Hematol Malig Rep. 2018;13(5):369-382. doi:10.1007/s11899-018-0466-6 7. Wang GG, Konze KD, Tao J. Polycomb genes, miRNA, and their deregulation in B-cell malignancies. Blood. 2015;125(8):1217-1225. doi:10.1182/blood-2014-10-606822 8. Mamessier E, Broussais-Guillaumot F, Chetaille B, et al. Nature and importance of follicular lymphoma precursors. Haematologica. 2014;9(5):802-810. doi:10.3324/haematol.2013.085548 9. Naradikian MS, Scholz JL, Oropallo MA, Cancro MP. Understanding B cell biology. In: Bosch X, Ramos-Casals M, Khamashta MA (eds.). Drugs Targeting B-Cells in Autoimmune Diseases. Basel, Switzerland: Springer; 2014:11-35. 10. Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22-33. doi:10.1038/nri2217 11. Mossadegh-Keller N, Brisou G, Beyou A, Nadel B, Roulland S. Human B lymphomas reveal their secrets through genetic mouse models. Front Immunol. 2021;12:683597. doi:10.3389/fimmu.2021.683597 12. von Keudell G, Salles G. The role of tazemetostat in relapsed/refractory follicular lymphoma. Ther Adv Hematol. 2021;12:1-8. doi:10.1177/20406207211015882.
